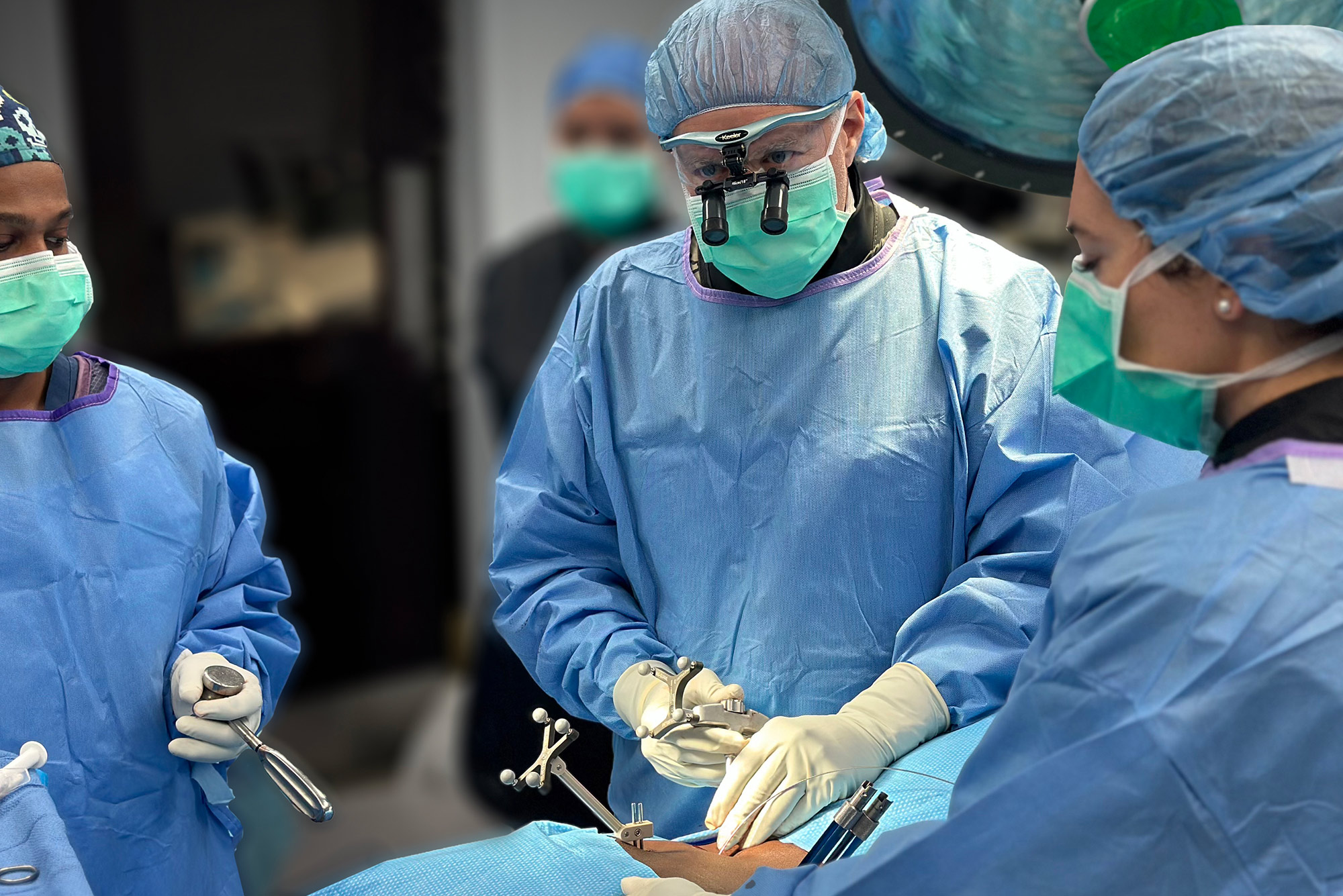
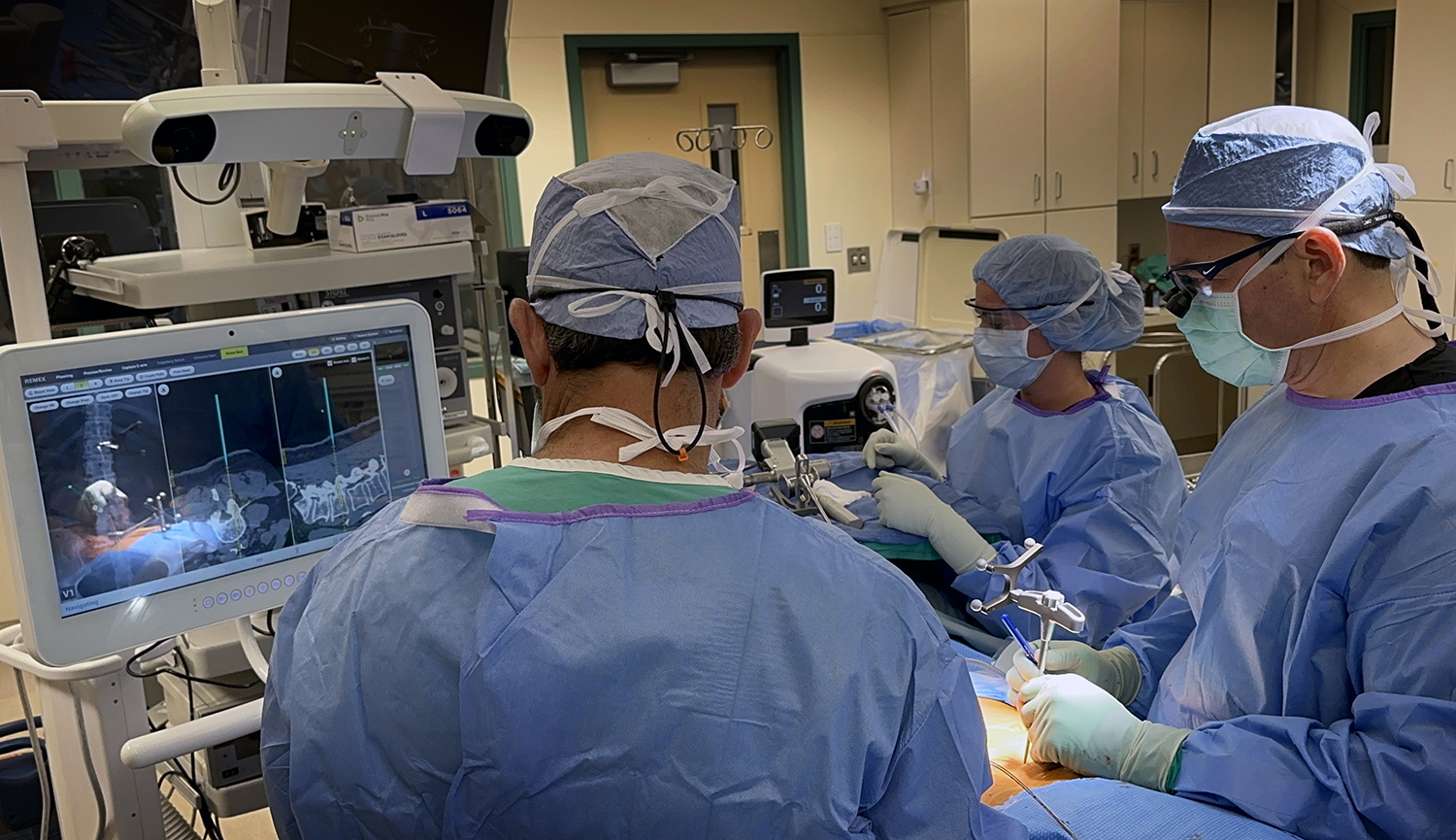
“Performing a sacroiliac joint fusion with spine navigation in an office setting while the patient was awake was a remarkable experience… doing this procedure with spine navigation removes any anatomical ambiguity within the SI joint while doing the procedure.”
Dr. Abdul Baker, an Emirati Neurosurgeon Practicing in Plano, TX, pioneers navigated minimally invasive SI fusion procedures for office-based surgery (OBS), maintaining patient safety while enhancing efficiency and reducing healthcare system costs.
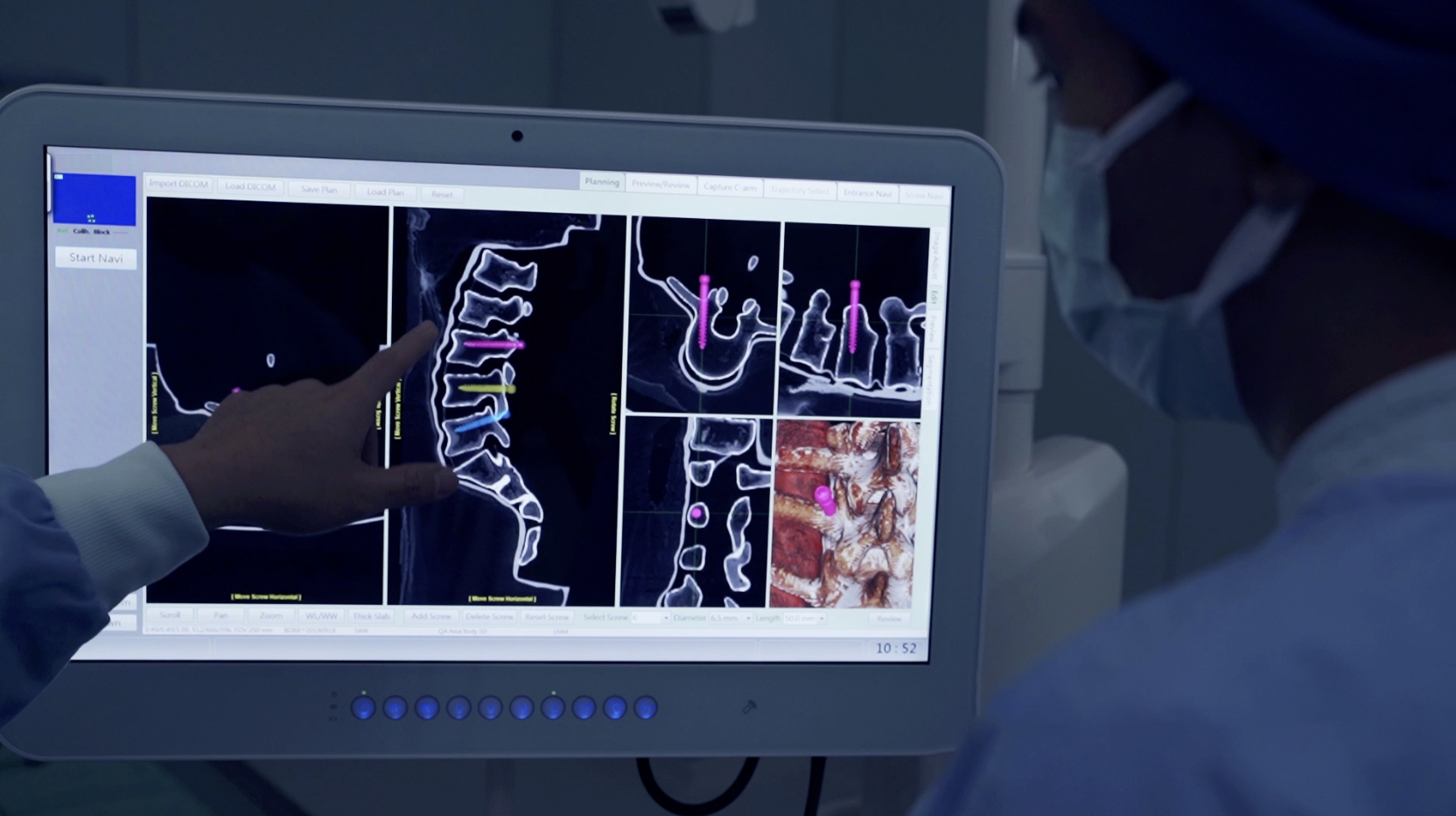
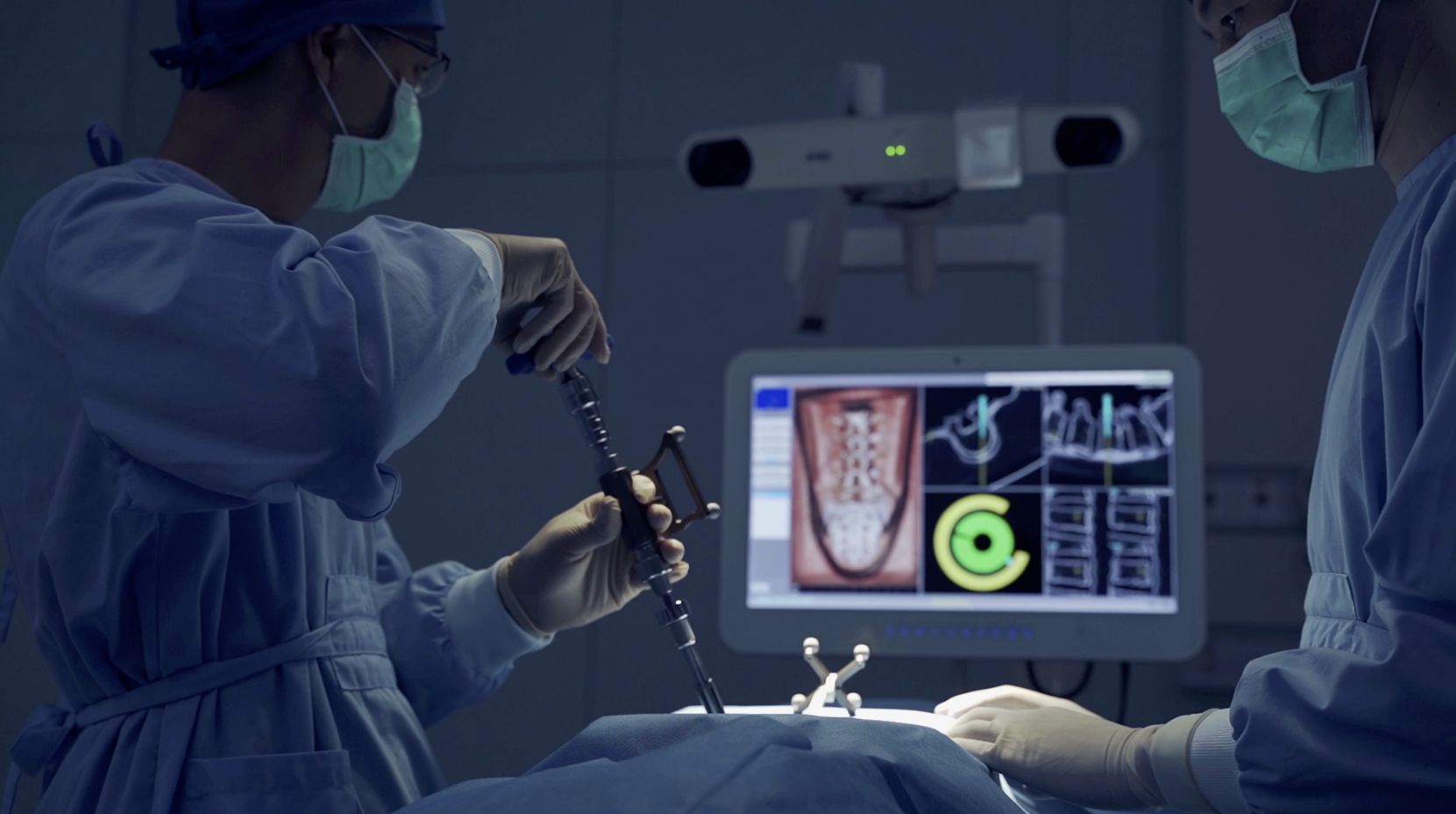
Plano, TX – July 2024—Neurosurgeon Dr. Abdul Baker, MD, FAANS, FACS, FCNS, and Captiva Spine has set a new benchmark in MIS spine surgery by performing their first navigated, office-based sacroiliac (SI) joint fusion using conscious sedation (patient awake). The procedure utilized Captiva Spine’s advanced WatchTower® ROAM™ Spine Navigation System and TransFasten-PSF Posterior SI Fusion System.
Setting New Standards in Spine Surgery
Minimally invasive spinal procedures aim to enhance the quality of care and lower the economic burden on the healthcare system by incurring less cost throughout the treatment of care. This office-based procedure marks a significant milestone in surgical navigation and heralds a new era in outpatient spine care, emphasizing clinical efficacy, safety, and economic stewardship.
Sacroiliac (SI) joint fusion connects the pelvis to the bottom of the spine, known as the sacrum. This joint often suffers from dysfunction, causing debilitating pain. SI Fusion treatments can be invasive, but Dr. Baker’s office-based clinical approach using spine navigation offers a transformative solution in this case.
“Performing a sacroiliac joint fusion with spine navigation in an office setting while the patient was awake was a remarkable experience. The patient responded very well, and I look forward to making this a standard procedure in my practice,” said Dr. Baker. “While the SI fusion can be done using fluoroscopic imaging, performing this procedure with spine navigation removes any anatomical ambiguity within the SI joint. That alone ushers many opportunities for my patients, peers, and myself.”
By performing the SI joint fusion with surgical navigation and the patient awake (under light sedation), Dr. Baker minimizes general anesthesia risks to ensure a swift recovery. The procedure was efficient, and the patient was discharged soon after the operation.
Dale Mitchell, president of Captiva Spine, said, “We are proud to be part of this achievement. The WatchTower spine navigation technology demonstrates how surgical navigation can assist even the most capable surgeon to deliver the clinical and economical solutions their patients demand.”
WatchTower Spine Navigation Technology
The WatchTower Spine Navigation System by Captiva Spine is an advanced platform designed to streamline surgical workflows and enhance precision. WatchTower:
- Eliminates the need for facilities to purchase an intra-operative CT scanner – Making navigation technology applicable to smaller medical centers, Ambulatory Surgery Centers (ASC), and office-based budgets.
- Uses a proprietary registration process that preserves the potential for percutaneous procedures – Which may reduce operative morbidity.
- Virtually eliminates radiation exposure – Enhancing safety. ¹
A Breakthrough in Office-Based SI Joint Fusion
Dr. Baker used the TransFasten-PSF Posterior SI Fusion System for the SI joint fusion. TransFasten affords surgeons and patients:
- Posterior Approach – Potentially reducing operative time and Neurovascular complications.
- Posterior SI Fusion Site Preparation – Allows efficient joint preparation for fusion and press-fit implant placement.
- Stabilization of the SI Joint – The geometry of the TransFasten implant, which bridges across the SI joint, is designed to stabilize the joint without unduly disrupting anatomy and creating new pain generators.
- Structural Allograft Implant – A custom allograft implant includes a large trans-joint graft window to accommodate a biologic fusion.
About Dr. Abdul Baker

Dr. Baker Minimally Invasive Neurosurgery – Left to Right: Lauren Sharma NP-C; Dr. Abdul Baker, MD, FAANS, FACS, FCNS; Sara Amys PA-C
Dr. Abdul Baker is one of Texas’s most trusted neurosurgeons, known for his vast experience in the field, extensive training, and commitment to minimally invasive techniques. He performed the first robotic spine surgery in the Middle East, Africa, and Abu Dhabi in March 2022 and has performed SI joint surgery for the past 10 years. He holds a Doctorate of Medicine from Wright State University. He completed an internship at Cleveland Clinic/Fairview Hospital and a spine fellowship at the University of Louisville and Johns Hopkins University. Dr. Baker continues to advance his neurosurgical practice as a Fellow of the American Association of Neurologic Surgeons, Associate Fellow of the American College of Surgery, and a Fellow of the Congress of Neurologic Surgeons.
Beyond his surgical practice, Dr. Baker actively collaborates with industry leaders to develop innovative spinal technologies. His efforts ensure patients receive cutting-edge treatments. By integrating these technologies into his practice, Dr. Baker is advancing the future of spine surgery with more effective and less invasive options.
Get Enabling Technology in Your Office-Based Facility or ASC
For those interested in seeing the ASC-READY WatchTower Spine Navigation System in action, along with our range of cervical, lumbar, and SI fusion solutions, Captiva Spine® invites you to visit our website at www.captivaspine.com/spine-navigation to schedule a demonstration. We welcome inquiries from healthcare providers, surgical facilities, and distribution professionals. To get in touch with us directly, please email our team at info@captivaspine.com
About Captiva Spine, Inc.
Captiva Spine, founded in 2007, is a privately held medical device company that brings healthcare providers, distribution professionals, and surgical facilities together with innovative spinal care solutions. With a focus on quality, Captiva Spine strives to create reliable systems that provide clinical and economical solutions to the spine market.
Captiva Spine – Navigating Today.
__________
This Press Release is also featured on PrWeb.com
- Published online November 24, 2023; DOI: https://doi.org/10.3171/2023.9.SPINE23422












 To take advantage of Captiva Spine’s innovative technologies and incorporate them into your healthcare offerings, please don’t hesitate to contact us. We welcome inquiries from healthcare providers, surgical facilities, and distribution professionals. To get in touch with us directly, please email our team at info@captivaspine.com
To take advantage of Captiva Spine’s innovative technologies and incorporate them into your healthcare offerings, please don’t hesitate to contact us. We welcome inquiries from healthcare providers, surgical facilities, and distribution professionals. To get in touch with us directly, please email our team at info@captivaspine.com