Captiva Spine Introduces ASC-Ready WatchTower ROAM, a Compact, Mobile Spine Navigation System Following the Publication of a Prospective Randomized Comparative Clinical Study of Accuracy and Safety Data
Enhanced Precision, Safety, and ASC Economics Backed by Clinical Research.
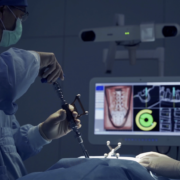
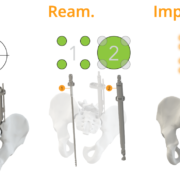
Jupiter, FL – May 2024 – Captiva Spine®, a medical technology organization connecting healthcare professionals, distribution specialists, and healthcare facilities with cutting-edge spinal care technology, recently received a US FDA 510(k)-clearance notice for the WatchTower® ROAM™. This new product is a compact version of their already ASC-READY WatchTower® SNS (Spine Navigation System) platform. The debut of the WatchTower ROAM follows closely after a recently published study in the Journal of Neurosurgery: Spine (JNS), affirming the effectiveness of the WatchTower Spine Navigation System and its novel 2D registration of a pre-operative CT for real-time 3D surgical navigation.
A Compact, Powerful, and Cost-Effective Spine Navigation System.
The WatchTower ROAM System allows spine surgeons who service multiple operative locations, especially Ambulatory Surgery Centers (ASC), to have a consistent navigation platform that can be relocated to various surgical sites within the community. This can be highly beneficial when a surgeon’s surgical volume is split between multiple locations, providing an economical alternative to acquiring a navigation system at every location. The WatchTower ROAM is designed to be more transportable.
Dale Mitchell, president of Captiva Spine, expressed his excitement about the new WatchTower ROAM, “the ROAM is a significant advancement towards navigation becoming a more practical standard of care through accessibility and affordability to previously underserved facilities. By integrating the real-time 3D spine navigation software used in the WatchTower SNS, ROAM can offer surgeons an affordable, efficient, powerful, and mobile device.”
In addition to the clearance of WatchTower ROAM, the FDA granted 510(k) clearance for calibrator compatibility with 9” and 12” C-arm image intensifiers, making the system compatible with more C-arms in more locations.
Accurate. Efficient. Reduced Radiation.
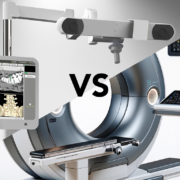
The WatchTower ROAM clearance follows closely after a study published in the Journal of Neurosurgery: Spine (JNS). The registration method and algorithm used in the WatchTower SNS system was the subject of a recent randomized study comparing it to an intra-operative CT scan-based system. A team led by Jau-Ching Wu MD, PhD, a Neurosurgeon at Taipei Veterans General Hospital and Assistant Professor at the National Yang-Ming University (et al.) has published a study in the Journal of Neurosurgery: Spine (JNS), Comparison of intraoperative cone-beam CT versus preoperative fan-beam CT for navigated spine surgery: a prospective randomized study,1 highlighting the clinical utility and benefits of the unique registration method compared to a commonly available intra-operative CT-based navigation platform. The study concludes that the preoperative CT-based spinal navigation system matches the accuracy and safety of the conventional CT-based intra-operative system. Additionally, it offers the advantages of a more rapid workflow and lower intra-operative radiation exposure.
The WatchTower SNS platform uses a novel 2D registration algorithm to register a pre-operatively taken CT scan using an AP and Lateral X-ray to provide real-time 3D surgical navigation.
Get Enabling Technology in Your Facility
For those interested in seeing the ASC-READY WatchTower Spine Navigation System in action, along with our range of cervical, lumbar, and SI fusion solutions, Captiva Spine® invites you to visit our website at www.captivaspine.com/spine-navigation to schedule a demonstration. We welcome inquiries from healthcare providers, surgical facilities, and distribution professionals. To get in touch with us directly, please email our team at info@captivaspine.com
About Captiva Spine, Inc.
Captiva Spine, founded in 2007, is a privately held medical device company that brings healthcare providers, distribution professionals, and surgical facilities together with innovative spinal care solutions. With a focus on quality, Captiva Spine strives to create reliable systems that provide clinical and economical solutions to the spine market.
Captiva Spine – Navigating Today.
__________
This Press Release is also featured on PrWeb.com
-
Published online November 24, 2023; DOI: https://doi.org/10.3171/2023.9.SPINE23422