MIS Pedicle Screw Systems Receive Additional Clearance For Larger Diameter Pedicle Screws
Captiva Spine’s MIS Pedicle Screw Systems Receive Additional FDA Clearance for Larger Diameter Pedicle Screws
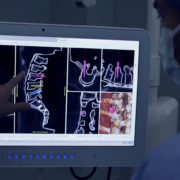
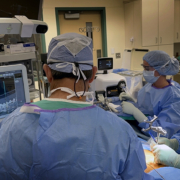
Captiva Spine, Inc., announced today that they have received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for larger diameter screws within their TowerLOX MIS Pedicle Screw System and CapLOX II Pedicle Screw System. The additions to their systems include 8.5mm and 9.0mm Polyaxial Screws (non-cannulated, cannulated and reduction) for MIS, Mini-Open and Open cases in 30mm-100mm lengths, while maintaining all of the same instrument compatibilities to the implant. The expansion of Captiva Spine’s line of cannulated and non-cannulated pedicle screws is an example of the company’s promise to continuously provide improvements and enhanced capabilities on behalf of surgeons and patients.
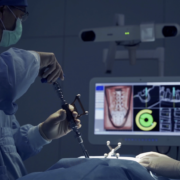
Dale Mitchell, President and Founder of Captiva Spine stated, “We are pleased to know that after successfully supporting over 5000 surgeries with Captiva Spine pedicle screws, that surgeons have come to know our company as providing thoughtful and elegant systems combined with reliable solutions for the growing and diverse clinical needs in spine care.” During the recent introduction of the newest MIS rod inserters, Captiva Spine’s Director of Research and Development, Dennis Ty, said “PivoQuik and PivoRod LP Inserters have been performing as expected, which has resulted in a bigger than expected demand for our reliable rod inserter tools and method.”
In addition to exhibiting at the AAOS (American Academy of Orthopaedic Surgeons) Annual Meeting this week (March 2-4) in Orlando, FL, Captiva Spine has recently updated their TowerLOX web page to include the system enhancements and a new animation to show the PivoQuik inserter in action (www.captivaspine.com/towerlox). Other additions to their website include information about bioactive glass and their newly distributed product, FIBERGRAFT® BG Morsels and Putty. Inquiries from tenured professionals looking to partner with a company that values integrity, commitment, and collaboration to build a relationship for the long run are always welcome. Captiva Spine can be contacted direct by phone at 561-277-9480 or via their website.
About Captiva Spine, Inc.
Captiva Spine is a privately owned medical device organization founded in 2007. Captiva Spine designs innovative medical and surgical apparatus, appliances, devices and instruments to support spine surgeons, tenured spine distributors, and healthcare facilities. They strive to provide patients with progressive spinal care devices with an obsessive focus on quality and they create and maintain sincere, honest, collaborative relationships. Valuing their relationships, above all else, fosters the mutual trust and openness needed for Captiva Spine to be a conduit of smart, elegant, and intuitive patient solutions. Captiva Spine consults with clients, providing valuable research information related to the design of surgical devices and instruments. Captiva Spine operates as a family of industry professionals that take pride in delivering these solutions responsibly and ethically while never losing sight of what they refer to as the Human Factor: Finding the joy in their daily lives and serving the needs of their customers with sincere, professional enthusiasm. Captiva Spine – Strength Through Connections.
This Press Release can be found on PR.com and is also featured with Becker’s Spine, Helio, and Orthopedic Design & Technology.